
Shells." Journal of Physical and Chemical Reference Data, volume 8, number 2, 1979, pp. 307–327. "Atomic Radiative and Radiationless Yields for K and L Hoboken, NJ: John Wiley & Sons, Inc, 2005. Introduction to Solid State Physics, 8th edition. "Why Helium Ends in “-ium”." Journal of Chemical Education, volume 81, number 7, 2004, p. 944. legacy/ english/ protection/ safework/ cis/ products/ icsc/ dtasht/ _icsc06/ icsc0604.htm. International Chemical Safety Card for Krypton (Liquefied, Cooled). New York: Dover Publications, Inc., 1984. New York: HarperCollins College Publishers, 1993. Inorganic Chemistry: Principles of Structure and Reactivity, 4th edition. "Critical Temperature of Elements and the Periodic System." Journal of Chemical Education, volume 50, number 5, 1973, pp. 335–336. "Thermal Conductivity of the Elements: A Comprehensive Review." Journal of Physical and Chemical Reference Data, volume 3, supplement 1, 1974, pp. I–1 to I–796. "From Dimer to Crystal: Calculating the Cohesive Energy of Rare Gas Solids." Journal of Chemical Education, volume 89, number 5, 2012, pp. 592–597. "A New Scale Of Electronegativity Ofĥ4 Elements Of Periodic Table Based On Polarizability Of Atoms." Journal of Theoretical and Computational Chemistry, volume 5, number 4, 2006, pp. 895–911. Table of Isotopes, 8th edition, volume 2. Oxford: Oxford University Press, 1998.įirestone, Richard B. Oxford: Oxford University Press, 2003.Įmsley, John. Nature's Building Blocks: An A-Z Guide to London: Taylor & Francis, 2002.Įmsley, John. Understanding the Properties of Matter, 2nd edition. Oxford: Oxford University Press, 1989.ĭe Podesta, Michael.
The Elements: Their Origin, Abundance and Distribution. "Covalent Radii Revisited." Dalton Transactions, number 21, 2008, pp 2832–2838. Platero-Prats, Marc Revés, Jorge Echeverría, Eduard Cremades, Flavia Barragán, and Santiago Alvarez. Cambridge: RSC Publishing, 2005.Ĭordero, Beatriz, Verónica Gómez, Ana E.

Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005. New York: Springer-Verlag New York, Inc., 2003.Ĭonnelly, Neil G., Ture Damhus, Richard M. "Atomic Screening Constants from SCF Functions." Journal of Chemical Physics, volume 38, number 11, 1963, pp. 2686–2689. "Explicit Periodic Trend of van der Waals Radii." The Journal of Physical Chemistry, volume 96, number 23, 1992, pp. 9194–9197. Berlin: Springer-Verlag, 1978.Ĭhauvin, Remi. The Atomic L Subshells." Atomic Data and Nuclear Data Tables, volume 85, number 2, 2003, pp. 291–315. "Fluorescence Yields and Coster–Kronig Probabilities for CalculationĪnd Conversion to Pauling Units." Journal of Chemical Education, volume 65, number 1, 1988, pp. 34–41. "Revised Mulliken Electronegativities: I. "Van der Waals Volumes and Radii." The Journal of Physical Chemistry, volume 68, number 3, 1964, pp. 441–451. Temperature on the International Temperature Scale of 1990 for a Selected Set of Secondary Reference Points." Metrologia, volume 33, number 2, 1996, pp. 133–154. "Binding Energies in Atomic Negative Ions: III." Journal of Physical and Chemical Reference Data, volume 28, number 6, 1999, pp. 1511–1533.īearden, J. Meteoritic and Solar." Geochimica et Cosmochimica Acta, volume 53, number 1, 1989, pp. 197–214. "The Definition of Electronegativity and the Chemistry of the Noble Gases." Journal of
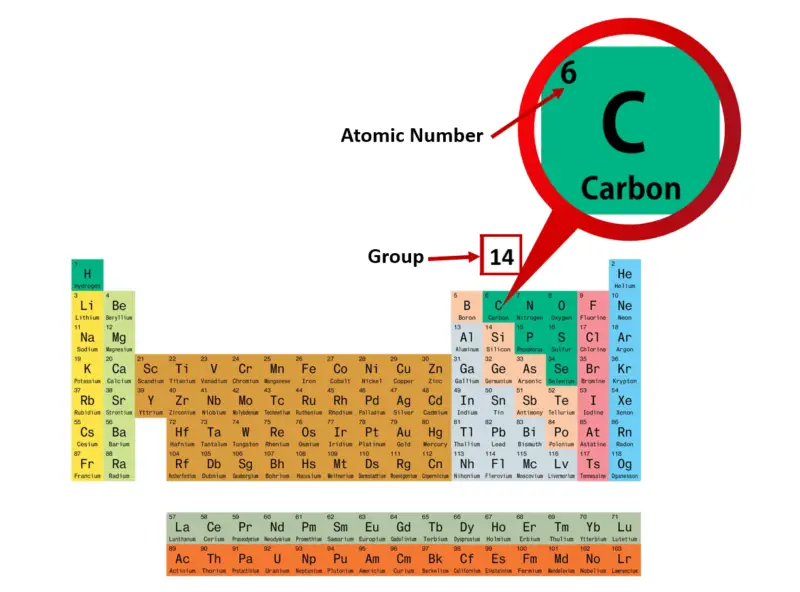
KRYPTON NUMBER OF VALENCE ELECTRONS FREE
"Electronegativity Is the Average One-Electron Energy of the Valence-Shell Electrons in Ground-State Free Atoms." Journal of New York: Oxford University Press, 1992.Īllen, Leland C. Next to a value above to see complete citation information for that entry)Īlbright, Thomas A., and Jeremy K.


 0 kommentar(er)
0 kommentar(er)
